Device allows robots to eat metal for energy
Researchers from the University of Pennsylvania’s School of Engineering and Applied Science have created a metal-air scavenger (MAS) that powers robots by eating metal. The new device bridges the gap between batteries and harvesters.
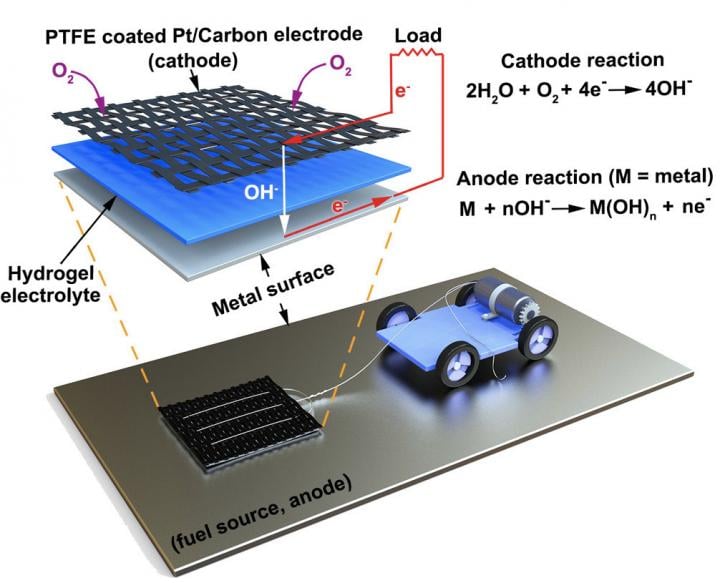 Like a traditional battery, the researchers' MAS starts with a cathode that's wired to the device it's powering. Underneath the cathode is a slab of hydrogel, a spongy network of polymer chains that conducts electrons between the metal surface and the cathode via the water molecules it carries. With the hydrogel acting as an electrolyte, any metal surface it touches functions as the anode of a battery, allowing electrons to flow to the cathode and power the connected device. Source: Pikul Research Group, Penn Engineering
Like a traditional battery, the researchers' MAS starts with a cathode that's wired to the device it's powering. Underneath the cathode is a slab of hydrogel, a spongy network of polymer chains that conducts electrons between the metal surface and the cathode via the water molecules it carries. With the hydrogel acting as an electrolyte, any metal surface it touches functions as the anode of a battery, allowing electrons to flow to the cathode and power the connected device. Source: Pikul Research Group, Penn Engineering
Batteries efficiently store energy, but they are heavy and have a limited supply. Harvesters collect energy from the environment, but they can only operate in certain conditions and cannot quickly turn energy into useful power. MAS works like a battery and a harvester. It provides power by repeatedly breaking and forming a series of chemical bonds. The power is supplied by the energy in the device’s environment, in this case, the chemical bonds in the metal and air surrounding MAS.
The researchers believe their device could be the basis for a new paradigm in robotics. Machines can stay powered by seeking out and eating metal and breaking down the chemical bond for energy in the same way that humans eat food for energy.
The team was inspired to create MAS because the technology that makes up robot brains and the technology that powers them are mismatched when miniaturized. When shrinking individual transistors, the chip could provide more computing power in a smaller package. But batteries do not work in the same way. With smaller batteries, the density of chemical bonds is fixed and there are fewer bonds to break. Sizing up the batteries does not fix this problem. Larger batteries are heavier and the robot has to overcompensate for the weight so any energy gained is wasted. The only way to break this is to forage for chemical bonds.
MAS starts with a cathode wired to the device is it is powering. There is a slab of hydrogel under the cathode that conducts electrons between the metal surface and cathode via water molecules. The hydrogel acts as an electrolyte so any metal surface it touches functions as the cathode of the battery and powers the device.
To test MAS, researchers connected MAS to a small motorized vehicle. The vehicle dragged the hydrogel behind it. The MAS vehicle oxidized the metallic surfaces it traveled over, leaving behind a layer of rust. The vehicle was outfitted with a small reservoir that continuously wicked water into the hydrogel to prevent drying. The team tested MAS on zinc and stainless steel metals and found that different metals give different energy densities depending on the materials’ potential for oxidation. Oxidation only happens within 100 microns of the surface, so the device has no significant risk of causing structural damage.
The researchers found that MAS had 13 times the energy density of a lithium-ion battery and the device only had to carry the hydrogel and cathode.
A paper on this device was published in ACS Energy.


Comments
Post a Comment